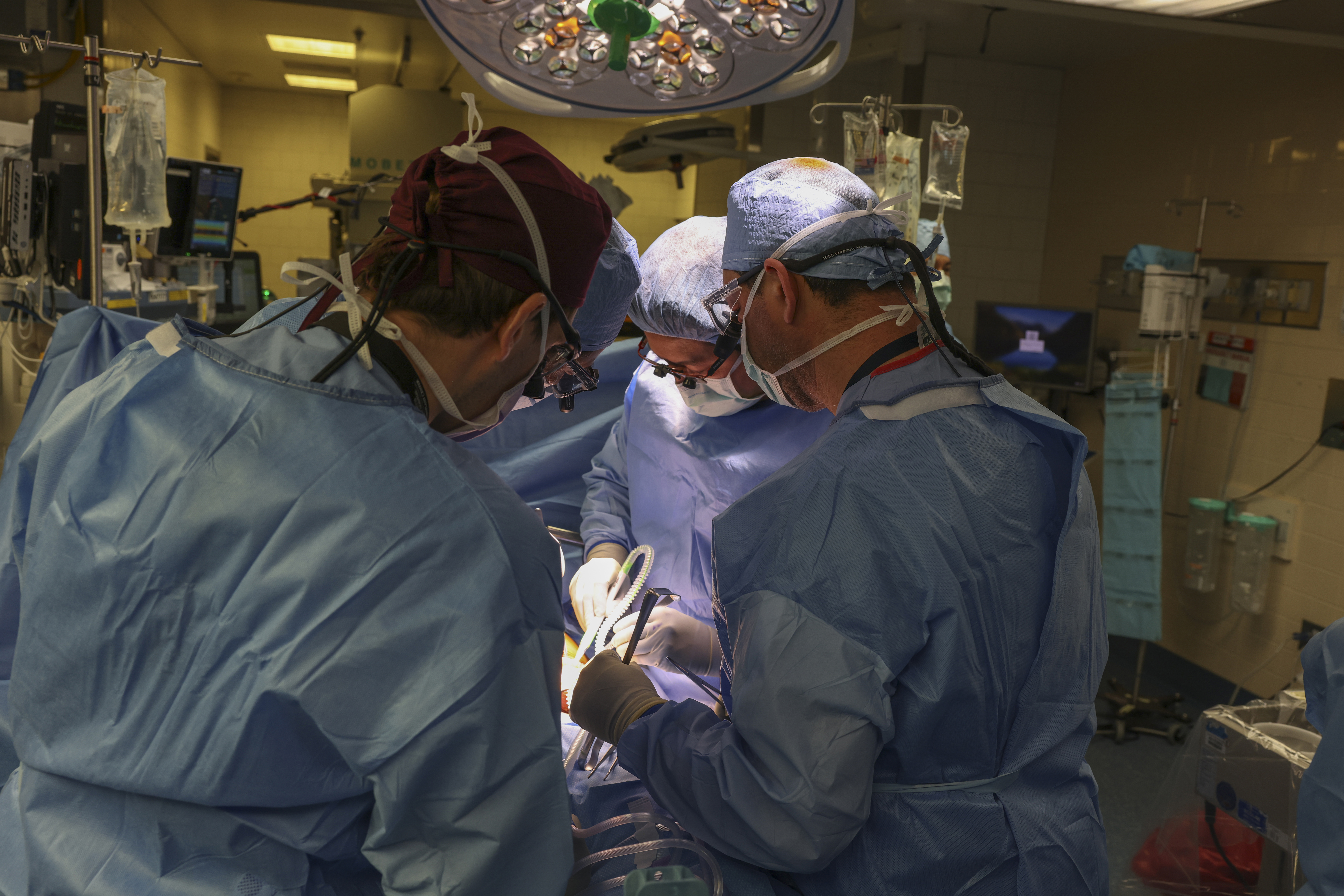
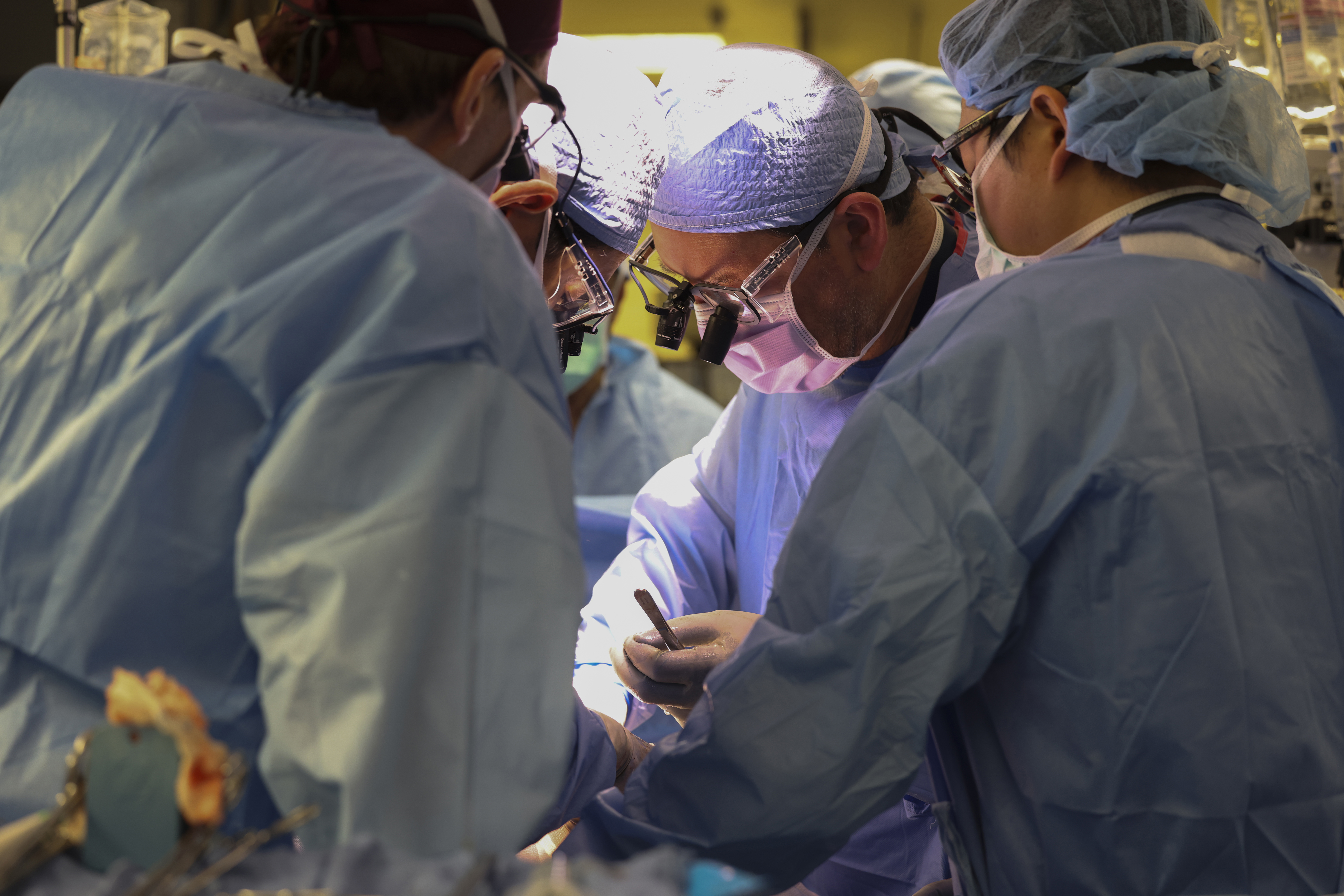
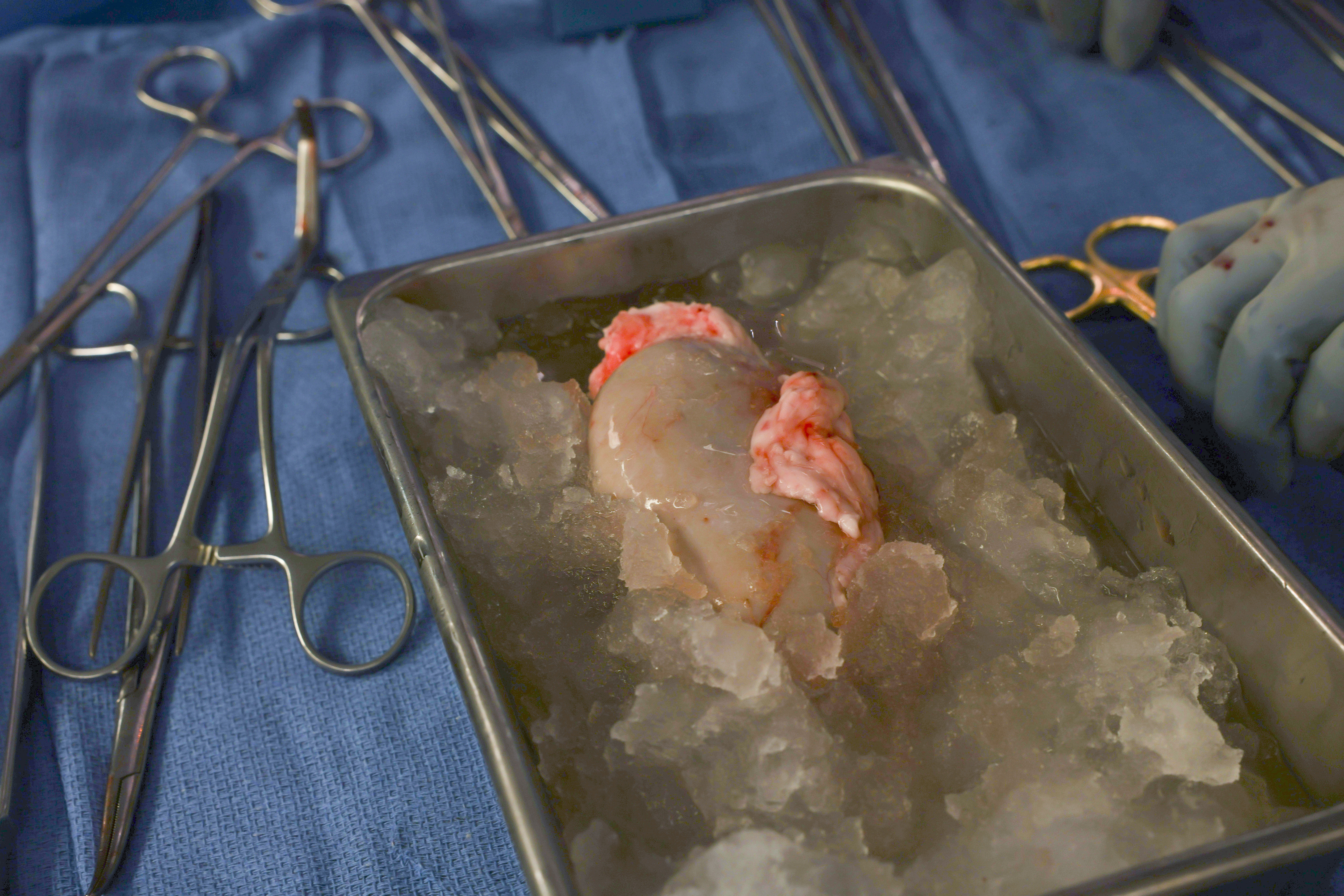
In a world first, we heard this month that US surgeons had transplanted a kidney from a gene-edited pig right into a residing human.
News stories mentioned the process was a breakthrough in xenotransplantation – when an organ, cells or tissues are transplanted from one species to a different.
Champions of xenotransplantation regard it as the answer to organ shortages the world over.
READ MORE: What the climate goes to be like over the Easter lengthy weekend
In December 2023, 1445 individuals in Australia have been on the ready listing for donor kidneys.
In the United States, greater than 89,000 are ready for kidneys.
One biotech CEO says gene-edited pigs promise "an unlimited supply of transplantable organs".
Not, everybody, although, is satisfied transplanting animal organs into people is de facto the reply to organ shortages, or even when it's proper to make use of organs from different animals this manner.
There are two important limitations to the process's success: organ rejection and the transmission of animal viruses to recipients.
But previously decade, a brand new platform and approach referred to as CRISPR/Cas9 – typically shortened to CRISPR – has promised to mitigate these points.
What is CRISPR?
CRISPR gene enhancing takes benefit of a system already present in nature.
CRISPR's "genetic scissors" advanced in micro organism and different microbes to assist them fend off viruses.
Their mobile equipment permits them to combine and in the end destroy viral DNA by chopping it.
In 2012, two groups of scientists found learn how to harness this bacterial immune system.
This is made up of repeating arrays of DNA and related proteins, referred to as "Cas" (CRISPR-associated) proteins.
When they used a specific Cas protein (Cas9) with a "guide RNA" made up of a singular molecule, they discovered they may program the CRISPR/Cas9 complicated to interrupt and restore DNA at exact places as they desired.
The system might even "knock in" new genes on the restore web site.
READ MORE: Where you’ll be able to – and may't – purchase alcohol over Easter
In 2020, the 2 scientists main these groups have been awarded a Nobel prize for his or her work.
In the case of the newest xenotransplantation, CRISPR know-how was used to edit 69 genes within the donor pig to inactivate viral genes, "humanise" the pig with human genes, and knock out dangerous pig genes.
A busy time for gene-edited xenotransplantation
While CRISPR enhancing has introduced new hope to the opportunity of xenotransplantation, even current trials present nice warning continues to be warranted.
In 2022 and 2023, two sufferers with terminal coronary heart illnesses, who have been ineligible for conventional coronary heart transplants, have been granted regulatory permission to obtain a gene-edited pig coronary heart.
These pig hearts had ten genome edits to make them extra appropriate for transplanting into people.
However, each sufferers died inside a number of weeks of the procedures.
Earlier this month, we heard a group of surgeons in China transplanted a gene-edited pig liver right into a clinically useless man (with household consent).
The liver functioned effectively up till the ten-day restrict of the trial.
How is that this newest instance completely different?
The gene-edited pig kidney was transplanted into a comparatively younger, residing, legally competent and consenting grownup.
The complete variety of gene edits edits made to the donor pig could be very excessive.
The researchers report making 69 edits to inactivate viral genes, "humanise" the pig with human genes, and to knockout dangerous pig genes.
Clearly, the race to remodel these organs into viable merchandise for transplantation is ramping up.
From biotech dream to scientific actuality
Only a number of months in the past, CRISPR gene enhancing made its debut in mainstream drugs.
In November, drug regulators within the United Kingdom and US authorized the world's first CRISPR-based genome-editing remedy for human use – a therapy for life-threatening types of sickle-cell illness.
The therapy, referred to as Casgevy, makes use of CRISPR/Cas-9 to edit the affected person's personal blood (bone-marrow) stem cells.
READ MORE: When does daylight saving finish?
By disrupting the unhealthy gene that offers pink blood cells their "sickle" form, the purpose is to provide pink blood cells with a wholesome spherical form.
Although the therapy makes use of the affected person's personal cells, the identical underlying precept applies to current scientific xenotransplants: unsuitable mobile supplies could also be edited to make them therapeutically useful within the affected person.
We'll be speaking extra about gene-editing
Medicine and gene know-how regulators are more and more requested to approve new experimental trials utilizing gene enhancing and CRISPR.
However, neither xenotransplantation nor the therapeutic purposes of this know-how result in adjustments to the genome that may be inherited.
For this to happen, CRISPR edits would should be utilized to the cells on the earliest levels of their life, comparable to to early-stage embryonic cells in vitro (within the lab).
In Australia, deliberately creating heritable alterations to the human genome is a felony offence carrying 15 years' imprisonment.
No jurisdiction on this planet has legal guidelines that expressly permits heritable human genome enhancing.
However, some international locations lack particular rules in regards to the process.
Is this the long run?
Even with out creating inheritable gene adjustments, nevertheless, xenotransplantation utilizing CRISPR is in its infancy.
For all of the promise of the headlines, there may be not but one instance of a steady xenotransplantation in a residing human lasting past seven months.
While authorisation for this current US transplant has been granted underneath the so-called "compassionate use" exemption, typical scientific trials of pig-human xenotransplantation have but to begin.
But the prospect of such trials would possible require important enhancements in present outcomes to realize regulatory approval within the US or elsewhere.
By the identical token, regulatory approval of any "off-the-shelf" xenotransplantation organs, together with gene-edited kidneys, would appear a way off.
This article written by Christopher Rudge, a regulation lecturer from the University of Sydney, is republished from The Conversation underneath a Creative Commons licence. Read the unique article right here.
Source: www.9news.com.au