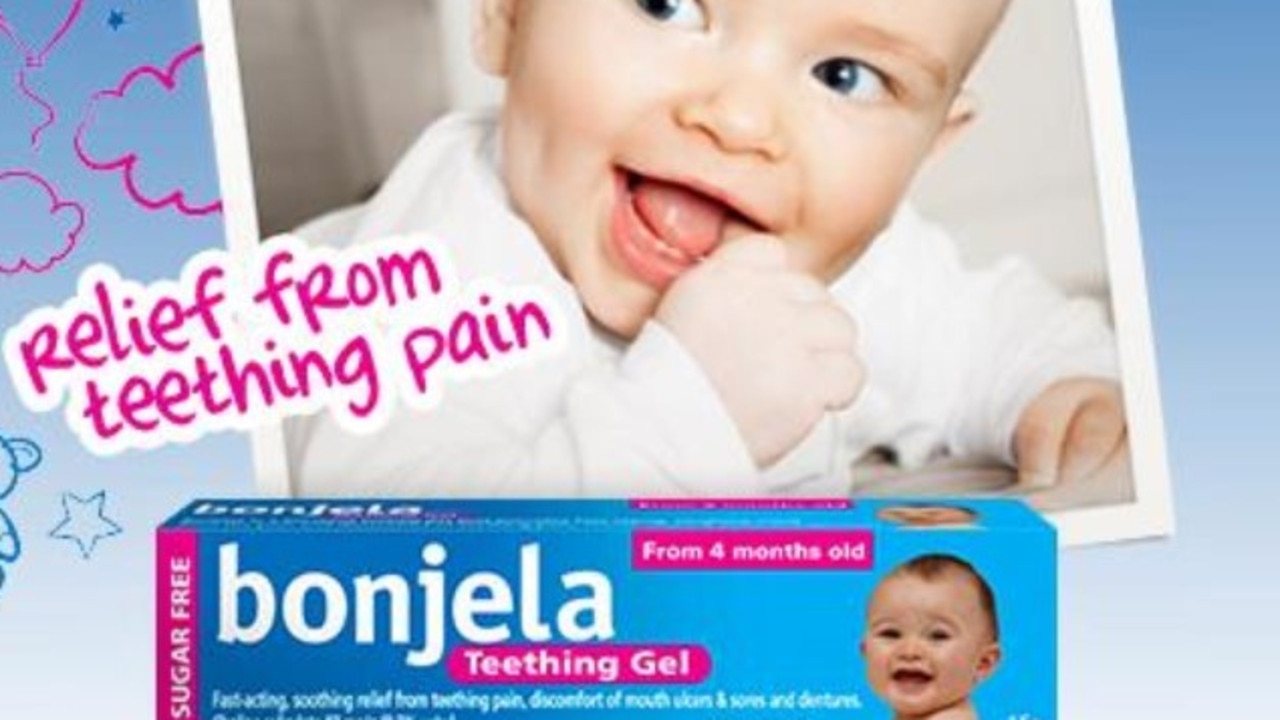
A typical treatment for teething and mouth ulcers will solely be obtainable from pharmacies from October, after the treatment watchdog modified its classification.
The Therapeutic Goods Association (TGA) has modified the categorisation of Bonjela Teething Gel and Bonjela Mouth Ulcer Gel from a Schedule 1 treatment to Schedule 2.
That means they’ll be pulled from grocery store cabinets over the following month, and the one technique to get them shall be from the chemist from October 1.
Coles and Woolworths have each confirmed they’ve ceased ordering the merchandise, and count on their present inventory to expire by the top of September.
The TGA change has come about over a long-held concern round Bonjela’s energetic ingredient, choline salicylate.
Choline salicylate is a non-steroidal anti-inflammatory (NSAID) which may result in an overdose if used excessively or incorrectly.
A TGA spokesperson says it’s generally utilized in different teething merchandise.
“Other products for the relief of pain, inflammation and discomfort associated with mouth ulcers/sores and new dentures or braces also contain choline salicylate,” the spokesperson stated.
“Substances in Schedule 2 may require advice from a pharmacist for safe use, which should be available from a pharmacy or, where a pharmacy service is not available, from a licensed person.
“From October 1, 2023, products containing choline salicylate for oromucosal use (administered via the mucus systems in the mouth) will be pharmacy medicines (Schedule 2).”
TGA treatment Schedules go all the best way to 10 — something Schedule 4 or above wants a prescription, and 10 is for substances that are dangerous and prohibited from sale or provide.
Originally printed as Common treatment to be pulled from cabinets amid overdose fears
Source: www.dailytelegraph.com.au